-
E-mail
46014616@qq.com
-
Phone
18028963555
-
Address
1st Floor, No. 3 Shugang Avenue, Hongmei Town, Dongguan City, Guangdong Province
Dongguan Dexiang Instrument Co., Ltd
Pharmaceutical industry electric hot blast drying oven
- Model
- Nature of the Manufacturer
- Producers
- Product Category
- Place of Origin


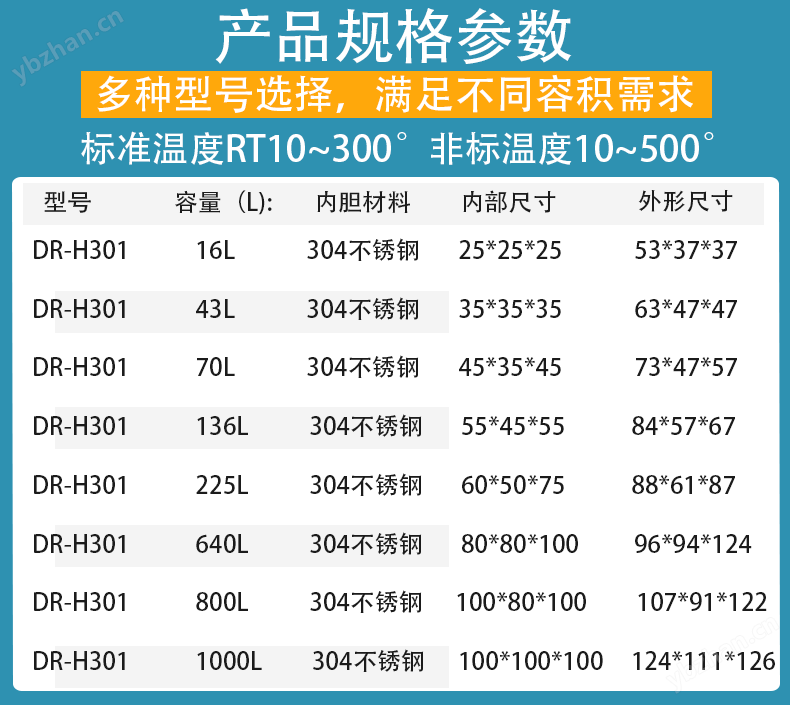







In pharmaceutical production, material drying and sterilization are key links to ensure product quality and safety. As one of the core equipment, the electric blast drying oven relies on itsHigh temperature uniformity, precise temperature control, and strong applicabilityIts characteristics are widely used in scenarios such as raw material processing, excipient drying, and intermediate sterilization. Starting from technical principles and combining with the actual needs of the pharmaceutical industry, this article exploresPharmaceutical industry electric hot blast drying ovenApplication logic and design essentials.
1、 The core technical principle of electric blast drying oven
Electric hot blast drying oven passes throughForced hot air circulation systemRealize efficient drying. Its core components include:
heating systemElectric heating tubes or heating plates heat the air to the set temperature.
Blast systemThe fan drives the hot air to form horizontal or vertical circulation inside the box, improving temperature uniformity.
Temperature control systemThe PID intelligent temperature controller adjusts the temperature in real time to ensure that the fluctuation range is within ± 0.5 ℃.
safety protectionMechanisms such as over temperature alarm and independent temperature limit protection prevent the risk of overheating.
questionWhy does the pharmaceutical industry have such strict requirements for temperature uniformity?
parseIn drug production, temperature deviation may lead to degradation of active ingredients, inadequate sterilization, or material clumping. For example, some thermosensitive raw materials are prone to decomposition at local high temperatures, and if the temperature distribution is uneven during the sterilization process, microorganisms may remain.
2、 Typical application scenarios in the pharmaceutical industry
Electric hot air drying oven covers multiple process links in the pharmaceutical industry. The following is a typical scenario analysis:
| scene | Technical Requirements | Key points of equipment configuration |
|---|---|---|
| Raw material drying | To avoid component decomposition, precise temperature control is required | Low temperature mode (60-120 ℃), low wind speed cycle |
| Sterilization of auxiliary materials | To kill microorganisms, F0 value needs to be verified | High temperature mode (160-180 ℃), multi-point temperature monitoring |
| Pre treatment of packaging materials | Remove moisture to prevent subsequent pollution | Rapid heating capability, large volume design |
| Intermediate aging test | Simulate storage conditions and verify stability | Program temperature control (multi-stage temperature curve) |
caseA certain pharmaceutical company adopts the method of drying freeze-dried powder injection excipientsDouble layer mesh structureSeparate materials and coordinateVertical air supplyThe mode effectively avoids dust cross pollution and shortens the drying cycle by 30%.
3、 Verification and compliance requirements
Pharmaceutical equipment must comply withGood Manufacturing Practice (GMP)andVerification GuidelinesRequirement: The validation of an electric blast drying oven typically includes the following steps:
Installation Confirmation (IQ)Check the equipment material (such as SUS304 stainless steel liner), sealing, and control system functionality.
Operational Qualification (OQ)Test the temperature distribution uniformity under no-load, half load, and full load conditions to ensure that the deviation at each point does not exceed ± 5 ℃ (sterilization process).
Performance Qualification (PQ)Simulate actual production loads to verify whether the drying/sterilization effect meets the process standards.
questionHow to choose a validation method suitable for pharmaceutical processes?
suggestionRegarding the sterilization process, it is necessary to focus on monitoringF0 value(Sterilization equivalent time), while the drying process needs attentionResidual moisture rateandenergy efficiency ratio.
4、 Key considerations for design selection
When pharmaceutical companies choose an electric blast drying oven, they need to consider the following factors:
temperature rangeConventional drying (60-150 ℃), high-temperature sterilization (160-250 ℃), or special requirements (such as vacuum assisted).
Cleanliness ClassIs a filtration system (such as HEPA filter) equipped to meet the requirements of a dust-free environment.
traceabilityDoes the data recording function support exporting temperature curves for easy audit tracking.
scalabilityIs there a reserved interface to connect to LIMS (Laboratory Information Management System).
promptFor multi variety and small batch production scenarios, it is recommended to choosemodular designEquipment that facilitates quick switching of process parameters.
5、 Daily maintenance and troubleshooting
Long term stable operation of equipment relies on scientific maintenance strategies:
cleaning frequencyClean the dust in the air duct every week to avoid blockages that may affect thermal efficiency.
calibration cycleCalibrate the temperature sensor every six months to ensure measurement accuracy.
Common FaultsFan abnormal noise (bearing wear), temperature runaway (heating tube aging), etc. need to be repaired in a timely manner.
caseA certain enterprise's neglect of air duct cleaning resulted in a decrease in drying efficiency. After investigation, it was found that the ventilation duct had accumulated dust up to 3cm thick, resulting in a 15% reduction in energy consumption after cleaning.
Conclusion
Pharmaceutical industry electric hot blast drying ovenThe application in the pharmaceutical industry is essentiallyDeep matching of process requirements and equipment performanceFrom temperature control accuracy to compliance verification, from scene adaptation to maintenance details, every step needs to be optimized based on actual production. With the development of intelligent technology, more devices may be integrated in the futureAdaptive control algorithmandRemote monitoring functionFurther enhance the reliability and efficiency of pharmaceutical production.




