-
E-mail
yang.yi@niumag.com
-
Phone
18516712219
-
Address
No. 97 Qinglian Road, Huguan Industrial Zone, Suzhou City
Suzhou Neway Analytical Instrument Co., Ltd
Application Background: Pain Points in Deep Sea Energy Development
With the surge in global energy demand, natural gas hydrates (commonly known as "combustible ice") are seen as strategic reserves for future energy due to their enormous storage potential. However, in order to achieve commercial mining, two major challenges must be addressed: thermodynamically induced decomposition and kinetic blockage. Traditional hydrate mining simulation experiments often rely on methods such as X-ray diffraction (XRD), differential scanning calorimetry (DSC), or high-pressure reactor observation. Although these methods can provide macroscopic data, they have significant shortcomings in microscopic phase evolution and in-situ dynamic monitoring.
In this context, low field nuclear magnetic resonance (LF-NMR) technology, with its unique physical mechanism, has become a key bridge connecting microscopic mechanisms and macroscopic characterization.
Core principle: The 'Dance' of Hydrogen Protons
The core of LF-NMR is to detect the magnetic resonance signal of hydrogen nuclei (¹ H) in substances. When the sample is placed in a constant magnetic field, hydrogen protons absorb radio frequency pulse energy at a specific frequency and resonate.
Relaxation Time
These are the core parameters for LF-NMR analysis of microstructure:
T1 (longitudinal relaxation): reflects the time for proton to recover magnetization strength.
T2 (transverse relaxation): reflects the time of proton release energy and phase loss.
Different states of water (such as free water, bound water, hydrate lattice water) have significantly different T2 relaxation times due to varying degrees of molecular motion restriction.
Signal difference
Free water: Strong fluidity, long T2 (millisecond level), slow signal attenuation.
Hydrate: Hydrogen atoms are bound in a rigid lattice, with extremely short T2 (microsecond level) and rapid signal disappearance.
By analyzing the area changes of different peak positions in the T2 spectrum, the amount of hydrate formation or decomposition can be quantitatively calculated.
Core application: Monitoring of thermodynamic accelerator regulation process
Adding thermodynamic promoters (such as methanol, ethylene glycol, surfactants, etc.) is a common anti blocking technique in hydrate mining. LF-NMR technology has demonstrated outstanding monitoring capabilities in such studies:
In situ dynamic monitoring: LF-NMR can be used to perform millisecond level time-resolved monitoring of hydrate formation/decomposition processes without damaging the sealing of high-pressure reaction vessels. Researchers can continuously record the change curve of T2 spectrum without taking out the sample, visually demonstrating how the promoter changes the phase transition rate.
Quantitative characterization and kinetic analysis: By comparing the T2 distribution curves of pure water systems and systems with different concentrations of accelerators added, the induction time, maximum generation rate, and equilibrium conversion rate can be accurately calculated. For example, in the tetrahydrofuran (THF) hydrate system, LF-NMR has been successfully used to track the distribution changes of THF between solution and hydrate.
Microscopic mechanism reveals that LF-NMR can not only see "how much" there is, but also "where" it is. It can distinguish between lattice water inside hydrates and free water outside, helping scientists understand how promoter molecules insert into the lattice surface of hydrates, thereby reducing the enthalpy change (Δ G) and accelerating the microscopic mechanism of decomposition.
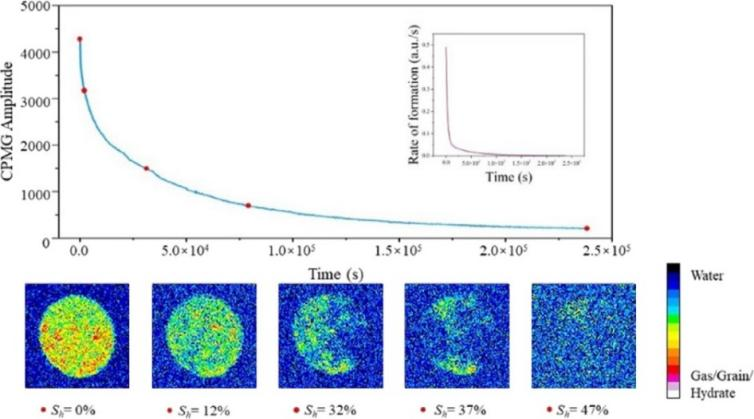
Figure 1: Nuclear magnetic signals at different stages of hydrate formation

Figure 2: Layered NMR signals at different stages of hydrate formation

Figure 3: T2 spectrum during hydrate formation process
Technical Comparison: LF-NMR vs Traditional Methods
Traditional detection methods:
X-ray diffraction (XRD): can only provide crystal structure information, cannot distinguish amorphous hydrates, and requires destructive sampling.
Optical microscope: limited to transparent samples, limited field of view, difficult to penetrate the interior of porous media.
DSC/DTA: Provides average thermal effect data, lacking spatial and temporal resolution.
Low field nuclear magnetic resonance (LF-NMR):
Non destructive: The sample can be reused and is suitable for long-term dynamic experiments.
High sensitivity: extremely sensitive to hydrogen containing fluids, capable of detecting trace hydrate formation.
Multidimensional information: Simultaneously providing chemical composition (T2 spectrum) and physical morphology (imaging) to achieve visible microscopic dynamics.
Low field nuclear magnetic resonance technology, with its non-destructive, fast, and highly sensitive characteristics, has become the first choice tool for studying the thermodynamic promoter regulation process of hydrates. It not only solves the problem of "in-situ monitoring" that is difficult to achieve with traditional methods, but also provides a new perspective for understanding the permeation mechanism of hydrates in porous media.