-
E-mail
yang.yi@niumag.com
-
Phone
18516712219
-
Address
No. 97 Qinglian Road, Huguan Industrial Zone, Suzhou City
Suzhou Neway Analytical Instrument Co., Ltd
Hydrate technology is currently a research hotspot in the fields of energy and environment, and the regulation process of metal nucleation promoters is a key link in improving the efficiency and stability of hydrate formation. How to monitor this complex process in real-time and without damage has always been a difficult point in scientific research and engineering practice. In recent years, low field nuclear magnetic resonance technology has become an important tool for monitoring the regulation process of hydrate metal nucleation promoters due to its unique advantages.
Application background of low field nuclear magnetic resonance technology
Traditional monitoring methods, such as optical microscopes and electrochemical sensors, can partially reflect the formation status of hydrates, but often cannot achieve non-invasive, real-time dynamic observation of the entire process, especially difficult to accurately capture micro phase transitions and material migration information under the action of metal nucleation promoters. With the continuous improvement of the mechanism and control requirements for hydrate formation, there is an urgent need for a technology that can penetrate deep into the material and provide molecular level information. Low field nuclear magnetic resonance technology highlights its application value in this context. It provides a unique window for studying the water state, pore structure, and phase transition process by detecting the relaxation behavior of hydrogen nuclei in water in a magnetic field.
Technical principle: Interpreting process information from hydrogen nuclear signals
The core principle of low field nuclear magnetic resonance technology is based on the nuclear magnetic resonance phenomenon of hydrogen nuclei (protons). In a constant magnetic field, hydrogen nuclei undergo energy level splitting and produce resonance signals under radio frequency pulse excitation. The speed of signal attenuation (relaxation), namely longitudinal relaxation time (T1) and transverse relaxation time (T2), is closely related to the degrees of freedom of water molecules, the chemical environment they are in, and their interactions with surrounding substances such as metal ions and nucleation interfaces. During the formation of hydrates, as water molecules transition from liquid water to cage shaped solid hydrates, their mobility sharply decreases, and the corresponding T2 relaxation time is significantly shortened. By monitoring the changes in T2 distribution in real-time, the nucleation and growth kinetics of hydrates can be accurately tracked, especially when metal ions (such as copper, nickel ions, etc.) are added as nucleation promoters in the system. This technology can clearly reveal the regulatory mechanism of metal ions on the structure, nucleation sites, and formation rate of water molecules.
Application in the regulation of hydrate metal nucleation promoter research
In specific research, low field nuclear magnetic resonance technology is directly used to monitor the entire process of hydrate formation in metal containing promoter systems. During the experiment, place the sample in a low field nuclear magnetic resonance analyzer for continuous or intermittent scanning. By analyzing the obtained T2 spectra, researchers can:
Identifying nucleation induction period: The initial change in T2 distribution indicates the onset of nucleation.
Quantitative phase transformation ratio: Calculate the amount of hydrate formation based on the T2 signal amplitude corresponding to hydrogen nuclei in free water and solid hydrates.
Elucidate the mechanism of action of the promoter: Compare the evolution differences of T2 spectra with and without metal promoters, clarify whether metal ions change the local hydration structure, provide more nucleation sites, or accelerate nucleation by affecting mass transfer processes. For example, certain metal ions may lead to an increase in the proportion of bound water, manifested as the appearance or enhancement of specific relaxation peaks on T2 spectra, which is directly related to their promoting effect.

Figure 1: Nuclear magnetic signals at different stages of hydrate formation
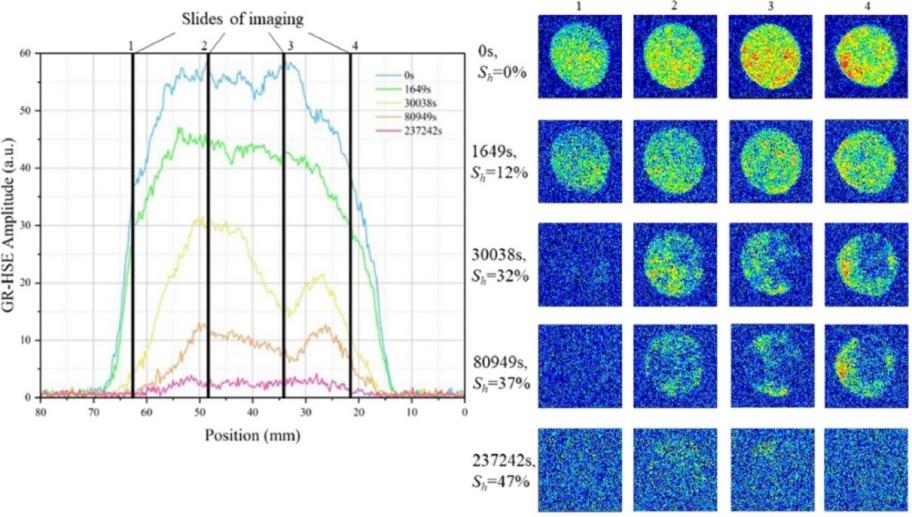
Figure 2: Layered NMR signals at different stages of hydrate formation

Figure 3: T2 spectrum during hydrate formation process
Comparative advantages with traditional methods
Compared to traditional detection techniques, low field nuclear magnetic resonance technology has shown significant advantages in this research field:
Non destructive in-situ monitoring: completely non-invasive, does not interfere with the nucleation process of the sample itself, and can achieve dynamic tracking of the same sample from start to finish.
Provide rich information: not only can it determine whether hydrates are formed, but it can also distinguish between free water, bound water, and water in hydrates, providing molecular level information on spatial distribution and state changes.
Zhuoyue's sensitivity: extremely sensitive to water phase transition, able to capture subtle changes in the early stages of nucleation, which is beneficial for studying the early regulatory behavior of accelerators.
Convenient operation and wide applicability: The equipment is relatively simple, with low requirements for sample preparation, suitable for various high-pressure and low-temperature reaction devices, and closer to actual process conditions.
In summary, low field nuclear magnetic resonance technology provides a powerful and unique research tool for a deeper understanding of the regulation process of hydrate metal nucleation promoters. It visualizes and quantifies micro dynamic processes that were previously difficult to observe, effectively promoting the optimization of hydrate formation technology and the development of control strategies. It has broad application prospects in natural gas hydrate extraction, carbon dioxide hydrate storage, and cold energy storage. With the continuous popularization and deepening of this technology, it will undoubtedly contribute more key insights to the development of energy and environmental science.