-
E-mail
yang.yi@niumag.com
-
Phone
18516712219
-
Address
No. 97 Qinglian Road, Huguan Industrial Zone, Suzhou City
Suzhou Neway Analytical Instrument Co., Ltd
With the increasing global demand for clean energy, natural gas hydrates (commonly known as "combustible ice") are seen as important strategic resources for the future due to their enormous reserves. However, hydrate extraction faces significant technological challenges - how to efficiently release methane gas without triggering submarine landslides or environmental pollution?
In this context, surfactants are widely used as efficient hydrate inhibitors and enhancers to reduce hydrate formation pressure, alter interfacial properties, and improve oil recovery. However, the dynamic adsorption and diffusion of surfactants in micro pores, as well as their real-time intervention in hydrate crystal growth, have always been elusive "black boxes". Traditional laboratory analysis often relies on offline sampling or destructive testing, which cannot capture the instantaneous changes in this microscopic regulation. Therefore, the scientific community urgently needs a non-invasive, real-time, and highly sensitive detection method.
Low field nuclear magnetic resonance (LF-NMR) technology is the key to solving this problem. The core principle is based on the magnetic resonance phenomenon of atomic nuclei. When the sample is placed in a constant magnetic field, the hydrogen protons (¹ H) inside will split into different energy levels. By applying radio frequency pulses of a specific frequency, these protons are excited and absorb energy to transition to high-energy states. Once the RF pulse stops, the proton will release energy back to a low-energy state with a specific time constant (relaxation time T1 or T2), producing a weak electromagnetic signal. In hydrate research, the difference in this signal is crucial:
Bound water: Water molecules strongly bound by hydrate cage like structures or rock pores, with extremely short relaxation time (T2 short) and fast signal attenuation.
Free water/oil: Unrestricted fluid with longer relaxation time (T2) and longer signal duration.
By analyzing these relaxation time spectra (T2 distribution), researchers can clearly distinguish the hydrate phase transition process, changes in pore fluid saturation, and microenvironmental changes after surfactant molecules enter the pores like a "listening diagnostic device".
Technical application: Real time monitoring of surfactant regulation process
LF-NMR technology plays an irreplaceable role in the regulation research of hydrate surfactant promoters:
1. Real time monitoring of phase transition kinetics
During the process of surfactant induced hydrate decomposition or inhibition, LF-NMR can capture the transition of water molecule states in the hydrate lattice in milliseconds. For example, when surfactants disrupt the cage like structure of hydrates, water molecules that were originally in a "bound state" quickly transition to a "free state", resulting in a sharp decrease in the area of the short relaxation peak in the T2 spectrum and a significant increase in the long relaxation peak. This real-time signal feedback provides precise data support for optimizing the concentration of surfactants.
2. Reveal the micro adsorption mechanism
Surfactant molecules typically have hydrophilic heads and hydrophobic tails, and they tend to adsorb at the water oil interface or water solid interface. LF-NMR can reveal whether surfactants have successfully entered nanoscale pores and altered the wettability of pore surfaces by measuring the distribution of fluids at different pore scales. This is crucial for evaluating its oil displacement efficiency in tight reservoirs.
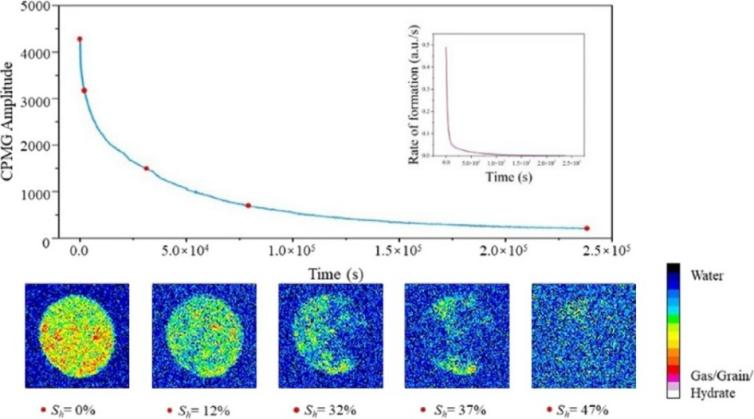
Figure 1: Nuclear magnetic signals at different stages of hydrate formation
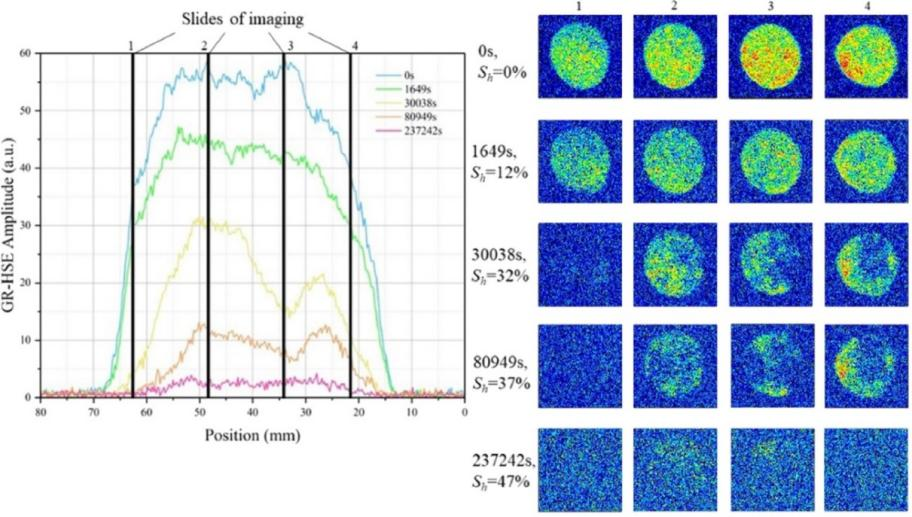
Figure 2: Layered NMR signals at different stages of hydrate formation
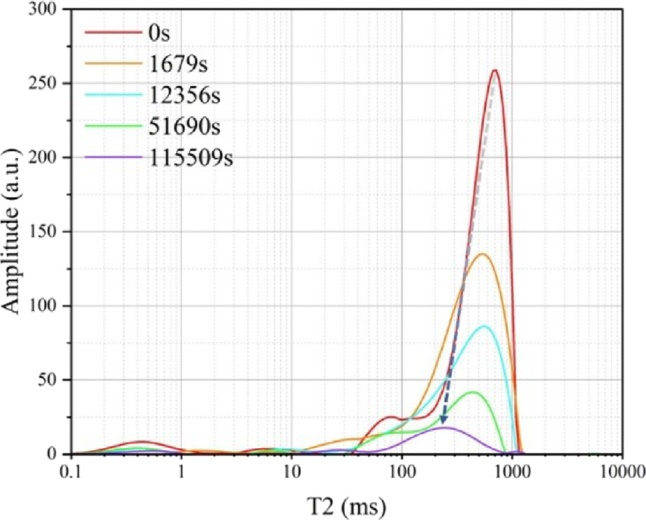
Figure 3: T2 spectrum during hydrate formation process
Advantage Comparison: Why Choose LF-NMR?
Compared to traditional detection methods, low field nuclear magnetic resonance technology has demonstrated overwhelming advantages in the study of hydrates and surfactants.
Traditional detection methods
Destructive: Methods such as centrifugation and Soxhlet extraction require the destruction of the sample structure, making it impossible to perform in-situ repeated measurements.
Long processing time: Pre treatment steps such as drying and heating may take several hours or even days, and cannot capture the instantaneous reaction process.
Single dimension: Often only a single component can be measured (such as only measuring water), making it difficult to simultaneously obtain information on multiphase fluids.
Low field nuclear magnetic resonance (LF-NMR)
Non destructive: The sample does not require any preparation, can be directly placed for testing, and can be repeatedly measured, preserving the original state of the interaction between the surfactant and the hydrate.
Rapid response: The testing speed is fast (in minutes), and even supports online continuous monitoring, accurately recording every moment of reaction.
Multidimensional characterization: T2 relaxation spectra can be obtained in one scan, while distinguishing water, oil, gas, and solid frameworks, providing rich microstructural information.
Low field nuclear magnetic resonance technology, with its non invasiveness, high precision, and rapidity, has become the first choice tool for monitoring the regulation process of hydrate surfactant promoters. It not only solves the pain point that traditional methods cannot observe micro dynamics, but also provides strong technical support for the safe and efficient exploitation of deep-sea natural gas hydrates.